There was a late evening lesson yesterday all the way until 7.30pm. It was a Biology practical session; and the tutor had us complete a short quiz of 10 True/False questions on the topic of diffusion. After having completed our laboratory experiment, the tutor went through the quiz before letting us off at 7.20pm.
One of the questions states that diffusion is the movement of particles from a region of higher concentration to a region of lower concentration. I picked False; and it turned out to be the correct answer. Diffusion - for the sake of clarity - is the NET movement of particles from a region of higher concentration to a region of lower concentration.
Another question states that diffusion takes place in a beaker of PURE WATER. I paused. This is tricky. By right, the particles all possess kinetic energy; and thus are moving about and bumping around randomly - and microscopically - at any given time. This indicates diffusion. But, on the other hand, this is a PURE solution we're talking about, and the water molecules are more or less evenly spaced out by default (and thus, there is no 'concentration gradient' to talk about in this scenario). I hesitated. Frowning slightly, I picked FALSE. And hoped for the best.
The answer is TRUE.
The whole class got it wrong.
Dr. C grinned knowingly. She was only too happy to be presented this opportunity to strengthen our foundation. "You see," She started, "this is one of the most common misconceptions that students have in Biology, even at JC or Uni level.
All particles possess kinetic energy, agree?" We nodded in agreement unanimously, and she continued, "Because of this kinetic energy, the particles are moving about and bumping into each other. How and why, then, can there be no diffusion occurring in a beaker of water molecules?" She asked.
"But diffusion is defined as the NET movement of particles down a concentration gradient, isn't it?" A fellow trainee asked.
"Yes, but this is the standard definition for diffusion. Look at the question again. Diffusion here, in this case, is asking about SIMPLE diffusion. The fact is plain and simple: Unless it's in a solid state where the molecules vibrate about it's fixed position, water molecules - in the state of liquid or gas - all possess kinetic energy, and thus, there will be diffusion.
Take for example, a stick of chocolate-vanilla ice cream. When the ice cream is in a solid state, the colours are separated clearly, isn't it? But when it goes into a liquid state, the colours get mixed together. By the same analogy, diffusion does not occur in ice; but there is diffusion in water, even though it's pure water.
Yes, all of you have been taught otherwise - that diffusion is the NET movement of particles. This is because it is the standard answer that is expected. Technically, though, it is fundamentally INcorrect. You need not explain to this level of depth to the kids at O Level standard, but it'll be good to clear this misconception that you guys here have all been 'brainwashed' into.
The underlying principle is: So long as kinetic energy is present, simple diffusion occurs."
My eyes widened in amazement. I've NEVER been asked whether does diffusion occur in a PURE solution. I might have thought about it once or twice, but only to brush it off after a while.
While Dr. C was explaining her case, something doesn't click.
From a Biology point of view, this case of argument is certainly right. In the solid state, water molecules only vibrate ABOUT THEIR FIXED POSITION. This is no moving about and bumping around of particles, and thus, it is right to state that diffusion does not occur in ice. In the liquid state though, water molecules move freely and thus there is diffusion. So, there is nothing wrong to state that there IS diffusion in a beaker of pure water.
From a Physics point of view, this case of argument seems a little flawed. I'm definitely not a Physics guru myself; but one thing I'm sure though: Energy is never destroyed, but gets converted from one form to another. So, based on this law, when two or more molecules bump into each other, doesn't a fraction of energy gets converted into something else? In the case of friction, part of the kinetic energy is lost in the form of heat, isn't it?
So, does it mean to say, if I leave a beaker of water TOTALLY untouched (so as to cut off any possible supply of kinetic energy) in TOTAL darkness and a lack of any elevation in temperature (so as to cut off any possible supply of heat energy) for millions and millions of years, will the water molecules gradually lose whatever existing kinetic energy they possess? So, then, will the water molecules gradually slow down their vibration/movement and, gradually, turn from water to ice - without any external interference? Hmmm...This is just, wrong, isn't it? Water doesn't turn into ice just like that.
Hmmm...
P.S. I had wanted to pose this question to Dr. C. But, you know, I do want to go home early one leh. Kekeke!
Thursday, 29 October 2009
Subscribe to:
Post Comments (Atom)















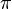


No comments:
Post a Comment